Eggbuckland Community College
Year 11 – 12 Chemistry Transition Questions
Q1.This question is about atoms and isotopes.
(a) Atoms contain protons, neutrons and electrons.
A lithium atom has the symbol
Explain, in terms of sub-atomic particles, why the mass number of this lithium atom is 7.
(3)
(b) Amounts of substances can be described in different ways.
Complete the sentences.
One mole of a substance is the relative formula mass in
......
The relative atomic mass of an element compares the mass of an atom of an element with the mass of an atom of
......
(2)
(c) Two isotopes of oxygen are and
Describe the similarities and differences between the isotopes and
You should refer to the numbers of sub-atomic particles in each isotope.
(3)
(Total 8 marks)
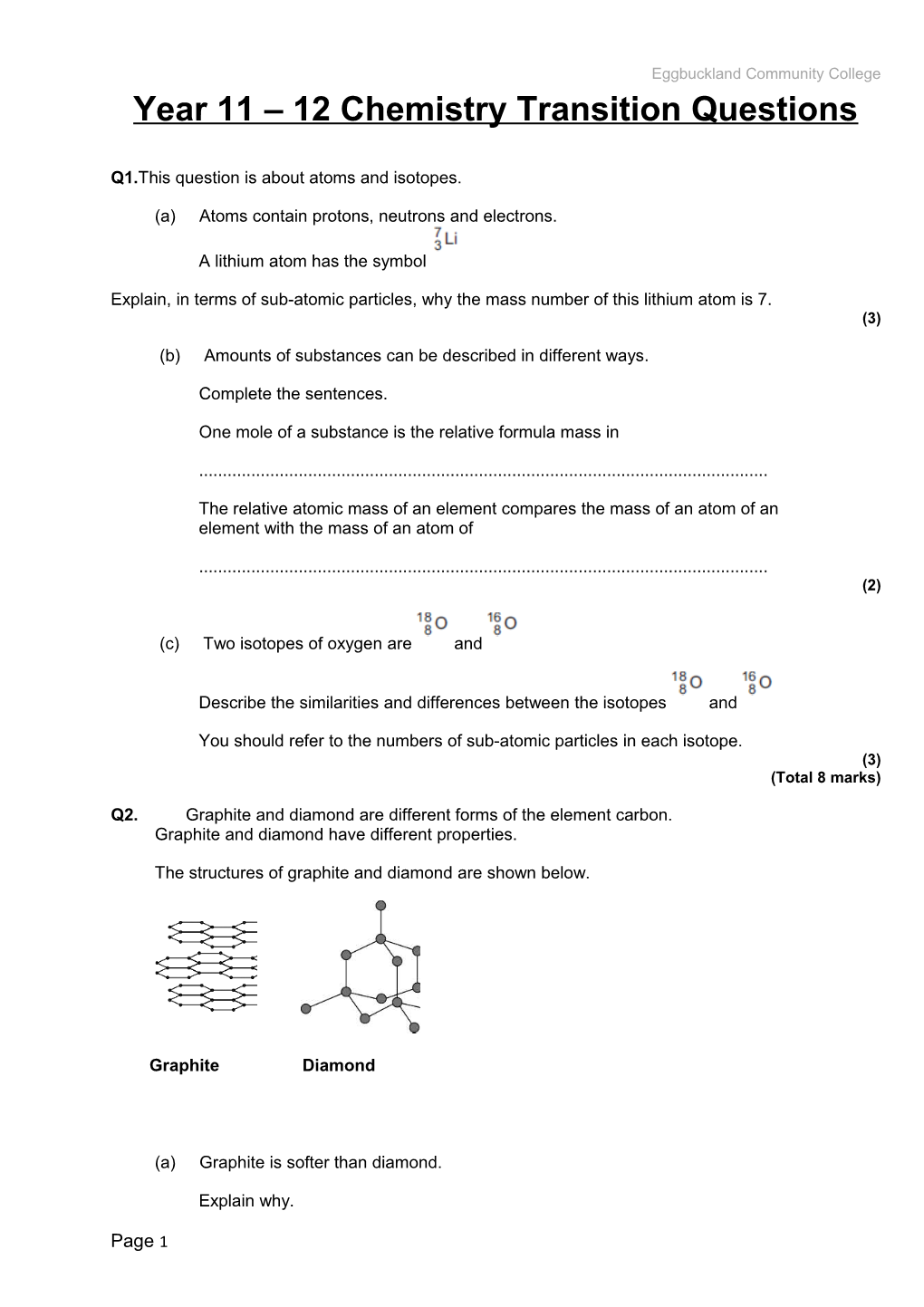
Q2. Graphite and diamond are different forms of the element carbon.
Graphite and diamond have different properties.
The structures of graphite and diamond are shown below.
Graphite / Diamond(a) Graphite is softer than diamond.
Explain why.
(4)
(b) Graphite conducts electricity, but diamond does not.
Explain why.
(3)
(Total 7 marks)
Q3. This question is about calcium hydroxide.
Ancient artworks and monuments can be protected from acid rain if the surface is sprayed with calcium hydroxide nanoparticles.
By Svilen Enev (Own work) [GFDL or CC-BY-SA-3.0], via Wikimedia Commons
(a) Calcium hydroxide has the formula Ca(OH)2
Why are there two hydroxide ions for each calcium ion in the formula?
(1)
(b) The calcium hydroxide is used in the form of nanoparticles.
What are nanoparticles?
(1)
(c) A student added water to calcium oxide to make calcium hydroxide.
The equation for the reaction is shown below.
CaO + H2O → Ca(OH)2
Calculate the maximum mass of calcium hydroxide which could be made from 2.00 g of calcium oxide.
Relative atomic masses (Ar): H = 1; O = 16; Ca = 40.
Maximum mass of calcium hydroxide = ...... g
(3)
(Total 5 marks)
Q4. Aspirin tablets have important medical uses.
(a) Aspirin is made when salicylic acid reacts with ethanoic anhydride.
The equation for this reaction is:
C7H6O3 + C4H6O3 → C9H8O4 + CH3COOH
salicylic acid aspirin
Calculate the maximum mass of aspirin that could be made from 100 g of salicylic acid.
Show clearly how you work out your answer.
The relative formula mass (Mr) of salicylic acid (C7H6O3) is 138.
The relative formula mass (Mr) of aspirin (C9H8O4) is 180.
Maximum mass of aspirin = ...... g
(2)
(b) (i)In an experiment a chemist calculated that the maximum yield of aspirin is 400 g.
The chemist did the experiment but only made 250 g of aspirin.
Calculate the percentage yield of aspirin for this experiment.
Show clearly how you work out your answer.
Percentage yield of aspirin = ...... %
(2)
(ii) Suggest one possible reason why the chemist did not have a percentage
yield of 100%.
(1)
(c) The use of a catalyst might reduce costs in the industrial production of aspirin.
Suggest how.
(1)
(Total 6 marks)
Q5. The picture shows a painting which was painted in a cave in France about 17 000 years ago.
By Carla Hufstedler [CC-BY-SA-2.0], via Wikimedia Commons
One of the pigments in this painting contains:
70 % of iron (Fe) and 30 % of oxygen (O)
Calculate the simplest (empirical) formula of this substance.
Relative atomic masses: O = 16; Fe = 56.
(4)
(Total 4 marks)
Q6.Glass is made from silicon dioxide.
© Velirina/iStock/Thinkstock
(a) Silicon dioxide has a very high melting point.
Other substances are added to silicon dioxide to make glass. Glass melts at a lower temperature than silicon dioxide.
Suggest why. (1)
(b) Sodium oxide is one of the substances added to silicon dioxide to make glass.
(i) Sodium oxide contains Na+ ions and O2– ions.
Give the formula of sodium oxide.
(1)
(ii) Sodium oxide is made by heating sodium metal in oxygen gas.
Complete the diagram to show the outer electrons in an oxygen molecule (O2).
(2)
(c) Glass can be coloured using tiny particles of gold. Gold is a metal.
Describe the structure of a metal.
(3)
(Total 7 marks)
Q7.Spacecraft have been to the planets Venus and Mars. The spacecraft have sent back information about the atmosphere of each planet.
© Tristan3D/Shutterstock
(a) The main gas in the atmosphere of Mars is carbon dioxide.
Explain why, in terms of structure, carbon dioxide is a gas, even at low temperatures.
(3)
(b) Gas chromatography linked to a mass spectrometer (GC-MS) is used to identify substances found on Mars.
(i) What is the purpose of gas chromatography? (1)
(ii) What information do the molecular ion peaks from the mass spectrometer give about the substances?
(1)
(c) The atmosphere on Venus contains droplets of sulfuric acid solution.
(i) Suggest a pH value for sulfuric acid solution.
pH = ......
(1)
(ii) Name the ion which makes sulfuric acid solution acidic.
(1)
(d) The atmosphere of Venus contains the isotopes and
Describe the similarities and the differences in the isotopes and
You should refer to the sub-atomic particles in each isotope.
(3)
(Total 10 marks)
Q8.This question is about sodium chloride and iodine.
(a) Describe the structure and bonding in sodium chloride.
(4)
(d) The bonding in iodine is similar to the bonding in chlorine.
(i) Complete the diagram below to show the bonding in iodine.
Show the outer electrons only.
(2)
(ii) Explain why iodine has a low melting point.
(3)
(iii) Explain, in terms of particles, why liquid iodine does not conduct electricity.
(2)
(Total 11 marks)
Q9.Aluminium has many uses.
(a) An aluminium atom has 13 electrons.
(i) Draw the electronic structure of an aluminium atom.
(1)
(ii) Name the two sub-atomic particles in the nucleus of an aluminium atom.
...... and ...... (1)
(iii) Why is there no overall electrical charge on an aluminium atom? (1)
(b) Rail tracks are made from steel. Molten iron is used to weld rail tracks.
The reaction of aluminium with iron oxide is used to produce molten iron.
(i) Balance the chemical equation for the reaction.
(1)
(ii) Why does aluminium react with iron oxide?
(1)
(Total 5 marks)
Q10. Crude oil is a mixture of hydrocarbons. Most of these hydrocarbons are alkanes.
(a) The general formula of an alkane is CnH2n+2
Complete the structural formula for the alkane that has six carbon atoms in its molecules.
(1)
(b) The boiling points of alkanes are linked to the number of carbon atoms in their molecules.
(i) Describe the link between the number of carbon atoms in an alkane molecule and its boiling point. (1)
(ii) Suggest two reasons why all of the alkanes in the bar chart are better fuels than the alkane with the formula C30H62
(2)
(c) During the last 200 million years the carbon cycle has maintained the percentage of carbon dioxide in the atmosphere at about 0.03 %.
Over the last 100 years the percentage of carbon dioxide in the atmosphere has increased to about 0.04 %.
Most of this increase is caused by burning fossil fuels to heat buildings, to generate electricity and to power our transport.
Fossil fuels contain carbon that has been locked up for millions of years.
(i) Burning fossil fuels, such as petrol, releases this locked up carbon. Balance the chemical equation for the combustion of one of the alkanes in petrol.
2 C8H18 / + / 25 O2 / / ...... CO2 / + / ...... H2O(1)
(ii) Where did the carbon that is locked up in fossil fuels come from?
(1)
(iii) The burning of fossil fuels has caused the percentage of carbon dioxide in the atmosphere to increase to above 0.03 %.
Explain why.
(2)
(Total 8 marks)
Q11. (a) Balance these chemical equations.
(i) H2 + O2 → H2O
(1)
(ii) Al + O2 → Al2O3
(1)
(b) Briefly explain why an unbalanced chemical equation cannot fully describe a reaction.
(2)
(Total 4 marks)
Q12. This information was taken from a label on a packet of crisps.
Main ingredients:Potatoes, vegetable oil, Worcester sauce flavour,
colourings, flavourings, salt.
Nutritional information (per 100 g):
Energy / 2040 kJ
Protein / 6.5 g
Carbohydrate / 55 g
of which sugars / 3 g
Fat / 27 g
of which saturates / 9 g
unsaturates / 18 g
Fibre / 4.5 g
Sodium / 1.2 g
Saturated fats are linked to heart problems. In order to claim that their crisps are healthy, the manufacturer keeps the proportion of saturated fats low.
(i) What type of fat contains double carbon carbon bonds?
(1)
(ii) The colour of bromine water is orange.
What is seen when bromine water is shaken with:
an unsaturated fat ......
a saturated fat? ......
(2)
(iii) Unsaturated vegetable oils can be hardened to make them useful as spreads. Describe how unsaturated vegetable oils are hardened.
(2)
(Total 5 marks)
Q13. Crude oil is a complex mixture of hydrocarbons, mainly alkanes. The number of carbon atoms in the molecules ranges from 1 to over 100.
(a) How does the boiling point change as the number of carbon atoms in the molecules increases?
(1)
(b) Name the method used to separate petroleum into fractions.
(1)
(c) The simplest hydrocarbon is methane, CH4. Its structure can be represented:
Draw the structure of ethane, C2H6.
(1)
(Total 3 marks)
Q14. The molecular formulae of two hydrocarbons M and N are given.
M = C4H10
N = C4H8
(a) M reacts with chlorine to form C4H9Cl.
(i) Write a balanced chemical equation for the reaction of chlorine with M.
(2)
(ii) Name this type of reaction.
(1)
(b) A displayed structural formula for N is:
Draw a displayed structural formula of a compound which is an isomer of N.
(1)
(c) Complete the boxes to show the displayed structural formula for each of the products formed.
(2)
(Total 6 marks)
Q15. Propane and ethene are both important hydrocarbons.
(a) Complete the table by adding the formula of the ethene molecule and the structure of the propane molecule.
(2)
(c) Ethene can be changed into a plastic. The equation shown below represents the reaction in which ethene is polymerised.
(i) What is the name of the plastic formed in this reaction?
(1)
(ii) What type of polymerisation reaction is shown in the equation?
(1)
(Total 4 marks)
Modem window frames are often made from uPVC which contains the plastic poly(chloroethene).
Replace your old wooden windows
with our super high quality uPVC
windows!
/
(a) State why plastic window frames need no painting or maintenance.
(1)
(b) Poly(chloroethene) is a polymer formed by the addition polymerisation of chloroethene.
(i) Chloroethene is an unsaturated molecule. Why is this molecule said to be unsaturated?
(1)
(ii) Complete the diagram to represent how poly(chloroethene) is formed from chloroethene.
(3)
(iii) Explain what is meant by the term polymerisation.
(2)
(Total 7 marks)
M1.(a) because this lithium atom has
3 protons
1
and 4 neutrons
1
mass number is total of neutrons and protons
accept protons and neutrons have a mass of 1
accept number of neutrons = 7 - 3(protons)
ignore mass of electron is negligible
1
(b) grams
accept g
1
12C
allow carbon-12 or C-12
ignore hydrogen or H
1
(c) any three from:
max 2 if no numbers given
numbers if given must be correct
• both have 8 protons
accept same number of protons
• 18O has 10 neutrons
• 16O has 8 neutrons
accept different number of neutrons or 18O has two more neutrons for 1 mark
• both have 8 electrons.
accept same number of electrons
3
[8]
M2. (a) Graphite:
because the layers (of carbon atoms) in graphite can move / slide
it = graphite
1
this is because there are only weak intermolecular forces or weak forces between layers
accept Van der Waals’ forces allow no covalent bonds between layers
1
Diamond:
however, in diamond, each carbon atom is (strongly / covalently) bonded to 4 others
allow diamond has three dimensional / tetrahedral structure
1
so no carbon / atoms able to move / slide
allow so no layers to slide or so diamond is rigid
1
(b) because graphite has delocalised electrons / sea of electrons
allow free / mobile / roaming electrons
1
which can carry charge / current or move through the structure
1
however, diamond has no delocalised electrons
accept however, diamond has all (outer) electrons used in bonding
1
[7]
M3. (a) because calcium is +2 and hydroxide is –1
accept to balance the charges
or
to make the compound neutral (in terms of charges)
allow calcium needs to lose 2 electrons and hydroxide needs to gain one electron
1
(b) particles of size 1-100 nm
allow clear comparison to ‘normal’ size particles
or particles with a few hundred atoms / ions
or particles with a high surface area (to volume ratio)
or as different properties to ‘normal’ size particles of the same substance
1
(c) Mr CaO = 56
and
Mr Ca(OH)2= 74
1
2/56 (x74) or 0.036 (x74)
or
allow ecf from step 1
74/56 (x2) or 1.3(214…) (x2)
1
2.6(428…) in range 2.6 to 2.96
correct answer with or without working gains 3 marks
allow ecf carried through from step 1
ignore final rounding to 3
1
[5]
M4. (a) 130.4
accept 130 to 130.43478………
correct answer gains two marks with or without working
an answer of 131 would gain one mark.
if answer is not correct then:
moles of salicylic acid = 0.7 ...... (1 mark)
or
mass of aspirin = moles of salicylic acid x 180 (1 mark)
or
100 x (180/138) (1 mark)
2
(b) (i) 62.5%
accept 63%
correct answer gains two marks with or without working
if answer is not correct then:
250/400 x 100 (1 mark)
2
(ii) any one from:
• reversible reaction
accept not all of the reactant converted to product
• some of product lost
1
(c) use lower temperatures
or
less energy needed
allow product made faster or more product made in a given time
1
[6]
M5. 70/56 30/16
division by atomic mass
1
= 1.25 = 1.875
proportion
1
2 3
ratio (accept 1:1.5 / 4:6 / etc)
allow e.c.f from proportion if sensible attempt at step 1
1
Fe2O3
formula allow e.c.f from ratio if sensible attempt at step 1
allow correct formula with no working = 1 mark
1
[4]
M6.(a) weaker bonds
allow (other substances) react with the silicon dioxide
or
fewer bonds
ignore weaker / fewer forces
or
disruption to lattice
do not accept reference to intermolecular forces / bonds
1
(b) (i) Na2O
do not accept brackets or charges in the formula
1
(ii)
electrons can be shown as dots, crosses, e or any combination
2 bonding pairs
accept 4 electrons within the overlap
1
2 lone pairs on each oxygen
accept 4 non-bonding electrons on each oxygen
1
(c) lattice / regular pattern / layers / giant structure / close-packed arrangement
1
(of) positive ions or (of) atoms
1
(with) delocalised / free electrons
reference to incorrect particles or incorrect bonding or incorrect structure = max 2
1
[7]
M7.(a) has simple / small molecules
accept molecular covalent
1
the intermolecular forces / intermolecular bonds (are weak)
do not accept weak covalent bonds or reference to incorrect bonding
1
only need a small amount of energy to be overcome
accept only need a small amount of energy to separate the molecules
if no other mark awarded, allow it has a low boiling point for 1 mark
1
(b) (i) to separate
1
(ii) (relative) molecular mass
allow Mr / (R)MM / relative mass / mass of molecule / (R)FM
1
(c) (i) any pH value from 0 to 6.9
1
(ii) hydrogen
allow H+
ignore H / H2 / H–
1
(d) any three from:
• same number of protons
accept same atomic numbernumbers if given must be correct
• 2H has one neutron
• 1H has no neutrons
accept different mass number or different number of neutrons for 1 mark
ignore relative atomic mass
• same number of electrons
numbers if given must be correct
3
[10]
M8.(a) lattice / giant structure
max 3 if incorrect structure or bonding or particles
1
ionic or (contains) ions
1
Na+ and Cl-
accept in words or dot and cross diagram: must include type and magnitude of charge for each ion
1
electrostatic attraction
allow attraction between opposite charges
1
(b) hydrogen
allow H2
1
sodium hydroxide
allow NaOH
1
(c) any one from, eg:
• people should have the right to choose
• insufficient evidence of effect on individuals
• individuals may need different amounts.
allow too much could be harmful
ignore religious reasons
ignore cost
ignore reference to allergies
1
(d) (i) one bonding pair of electrons
accept dot, cross or e or − or any combination, eg
1
6 unbonded electrons on each atom
1
(ii) simple molecules
max 2 if incorrect structure or bonding or particles
accept small molecules
accept simple / small molecular structure
1
with intermolecular forces
accept forces between molecules
must be no contradictory particles
1
which are weak or which require little energy to overcome − must be linked to second marking point
reference to weak covalent bonds negates second and third marking points
1
(iii) iodine has no delocalised / free / mobile electrons or ions
1
so cannot carry charge
if no mark awarded iodine molecules have no charge gains 1 mark
1
[14]
M9.(a) (i) 2.8.3
any sensible symbol can be used to represent an electron
1
(ii) proton(s) and neutron(s)
both needed for the mark
1
(iii) number of protons is equal to number of electrons
allow positive and negative charges cancel out
allow same amount of protons and electrons
1
(b) (i) 2 Al + Fe2 O3 → 2 Fe + Al2 O3
equation must be balanced
1
(ii) aluminium is more reactive (than iron)
it = aluminium
accept converse
accept aluminium displaces iron
accept aluminium is higher in the reactivity series (than iron)
1
[5]
M10. (a) complete diagram with 2 carbon atoms and 5 hydrogen atoms each C–C
and each C–H linked by a single line (bond)
1
(b) (i) the greater the number of (carbon) atoms (in an alkane molecule) the
greater its boiling point or vice versa
allow as the (carbon) chain gets longer the boiling point increases
ignore melting points
do not accept reference to greater number of molecules
1
(ii) they = hydrocarbons from the graph
it = C30H62
any two from:
• low boiling point / volatile
accept they are gases or liquids
• low viscosity
• high flammability
accept easier to burn / ignite
• small molecules
accept short chains
ignore number of carbon atoms
• burn completely
ignore speed of burning
2
(c) (i) 16 (CO2) + 18 (H2O)
1
(ii) (carbon dioxide in the Earth’s early) atmosphere
accept from volcanoes (millions of years ago)
or from dead plants / animals
allow dead sea creatures
ignore shells
1
(iii) increase in burning / use of fossil fuels
1
locked up carbon (carbon dioxide) is released
allow carbon / carbon dioxide from millions of years ago is released
accept extra carbon dioxide is not ‘absorbed’ (by the carbon cycle)
1
[8]
M11. (a) (i) H2 + O2 → H2O *both circled correct
for 1 mark
1
(ii) A1 + O2 → A12O3 all circled correct
for 1 mark
1
(b) idea that:
must end up with the same number of atoms as at the start
any 2 each
otherwise matter is shown to be lost/gained
for 1 mark
won’t show correct amount of each element/compound
2
[4]
M12. (i) (poly)unsaturated
accept monounsaturated
1
(ii) (turns) colourless or colour disappears / decolourises
do not accept clear
1
stays the same colour / orange / no change
allow yellow-orange / orange-brown / red-orange
1
(iii) (react) with hydrogen / H2 / hydrogenation
1
any one from:
• heated / 60 °C
• catalyst / nickel
1
[5]
M13. (a) the more C atoms the higher the b.pt./temperature
Allow just higher. Not answer based on melting point
for 1 mark
1
(b) (fractional) distillation/fractionation
for 1 mark
1
(c)
must include H atoms and lines not CH3 – CH3
for 1 mark
1
[3]
M14. (a) (i) C4H10 + Cl2 → C4H9Cl + HCl
reactants
1
products
ignore incorrect balancing
no state symbols required
1
(ii) substitution / chlorination
1
(b)